BiCOM Obtains Health Canada Approval for Tonometer Diaton, Unique Glaucoma Eye Test Through Eyelid
BiCOM’s Diaton tonometer has been awarded with Canadian Government Certificate, Medical License of Health Canada #76513. Now Health Canada approval in addition to USA Food & Drug FDA and CE MARK for the European market opens many international doors for BiCOM Inc., a global distributor of advanced Diaton tonometer – unique tonometry (Glaucoma Eye Test) THROUGH THE EYELID.
BiCOM Inc. reveals an advanced trans-scleral and trans-palpebral Diaton tonometer (Glaucoma Eye Test) to Canada through its Canadian distributor Northern Optotronics (NOI) www.noi.ca .
Mr. Ross McDonald, Vice President of NOI and a 25-year veteran of the ophthalmic and medical device industries, after the introduction of Diaton tonometers at COS Meeting said, "In my 25 years of experience in the ophthalmic industry, this is the first time that I see so much interest in a new product. Tonometer Diaton will have great success in the market."
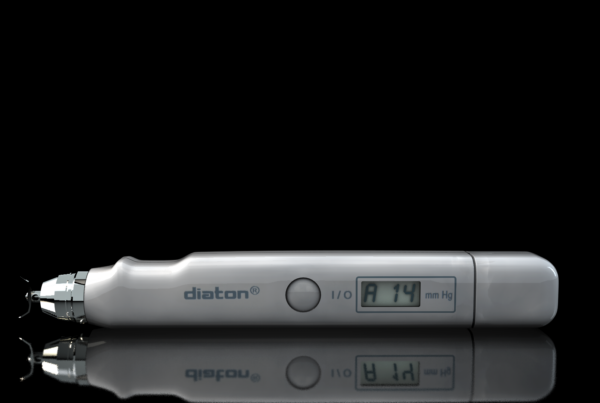
Diaton Tonometry is a unique approach to measuring intraocular pressure (IOP) THROUGH THE EYELID – no contact with the cornea, no anesthesia drops or sterilization is required. BiCOM’s pen-like, hand-held, portable diagnostic device is perfect for measuring eye pressure and helps in diagnosis and prevention of blindness caused by glaucoma. Tonometer Diaton is irreplaceable for mass glaucoma screenings in children and adults.
Canadian Government Certificate, Medical License – Health Canada #76513 in addition to USA Food & Drug FDA and CE MARK for the European market open many international doors for BiCOM Inc., a global distributor of advanced Diaton tonometers – unique tonometry through the Eyelid.
Diaton tonometry – a new trans-palpebral and trans-scleral methodology has received the Gold Medal at the International Exhibition of Research and new Technology in Geneva & the Gold Medal at the International Exhibition of Innovation Research and New Technology – “Brussels Eureca”.
Portability, safety and simplicity make Tonometer Diaton ideal for a wide range of applications: for mass glaucoma screening of the population, at the patient’s bedside, in geriatrics homes, in children hospitals, for the military and for home use.
Diaton Tonometer is intended for use by Inpatient & Outpatient Clinics such as Hospitals, Emergency Rooms, Nursing & Elderly Homes, General & Specialty Practitioners as well as Ophthalmologists and Optometrists.
Tonometer Diaton is the ideal solution in the following cases when the use of other devices is problematic or impossible:
* mass prophylactic screening of patients;
* IOP control during clinical observation of glaucoma patients;
* ortoclinostatical probe, as an additional test to diagnose glaucoma and during select the adequate hypotensive therapy;
* ophthalmotone monitoring (even at night time);
* IOP measuring during contact correction (lenses are not taken out),
* IOP measuring in immobilized patients;
* IOP measuring in children.
* on patients with the following conditions: chronic conjunctivitis, cornea pathology, including keratitis, keratotone, cornea dimness, after penetrating keratoplastics, keratoprosthesis, laser refractive correction of the eyesight, high degree of ametropy, astigmatism;
* on patients with medicinal allergies;
* Lasik/ PRK (recent clinical trials have proved that Diaton is the only device that can be used for IOP measurement right after these surgeries)
Diaton tonometer is a perfect device for mass screenings for glaucoma for any age group. Undiagnosed and untreated, glaucoma can cause blindness. Glaucoma is the leading cause of blindness in all age groups – from babies to senior citizens. Everyone needs to get diagnosed to preserve eyesight.
Besides determining the potential for glaucoma onset, Tonometer Diaton can also be used for monitoring treatment effectiveness throughout the day. Unlike other tonometers, Diaton can be used repeatedly during the day with no effect on the eye.
BiCOM Inc., had signed Northern Optotronics Inc. (NOI) as Diaton tonometers Canadian distributor http://www.noi.ca with representatives throughout Canada. NOI covers the full scope of customer service, which involves client training, follow ups, hotline support, sales support, etc. To get more information on the device or to make a purchase, visit the official Diaton website – www.tonometerdiaton.com, or call at the toll free number 1-877-diatons (877.342.8667) or a local number in Canada 1.416.726.1435
About Northern Optotronics (NOI)
Northern Optotronics Inc. (NOI), business is structured around the customer-driven priorities of cost-effectiveness, performance and support. Company services medical markets with concentration in Ophthalmology and Optometry. The company is located at Cambridge, Ontario and services most areas of Canada. More at http://www.noi.ca
About BiCOM Inc.
BiCOM, with its 15 years of tradition and global clientele, has proven to be the enterprise of the highest level of professionalism, integrity and financial solvency. A unique team of engineers, medical, legal and business experts makes BiCOM Inc. the right place for global talent to find support and guidance. BiCOM Inc. sees its mission in bringing to market innovative products developed and manufactured worldwide.
BiCOM represents and supports Diaton tonometer in over 30 countries and growing.
http://www.BiCOM-Ophthalmic.com