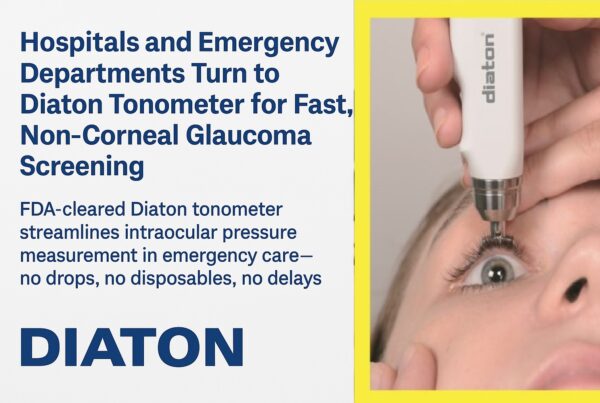
Diaton Tonometer is a unique & innovative tonometry instrument, unlike all others, allows to accurately measure Intraocular Pressure (IOP) through the Eyelid and Sclera, without touching the eyeball direct with Zero-contact with the cornea or influence of the cornea on Diaton IOP results. With Diaton there is no Risk of Infection or need for Sterilization as there is no contact with mucous membranes.
Diaton tonometer meets and exceeds Joint Commission Guideline for Disinfection and Sterilization Guidelines and Standards as Diaton has no contact with an eyeball or the mucous membrane.
The American Academy of Ophthalmology has reported that transmission of adenovirus and herpes simplex virus HIV, hepatitis C virus (HCV), enterovirus 70, Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, Acanthamoeba, and prions (transmissible spongiform encephalopathies, such as Creutzfeldt-Jakob disease) could occur from failure to adequately disinfect ophthalmology devices, such as tonometers.
The Joint Commission, Division of Healthcare Improvement in Issue 49, May 2019 noted that “Tonometer tips are particularly problematic because disinfectants can dissolve the glue that holds the hollow tip together, causing the tip to swell and crack. It’s important to note that tonometer tips have been identified as sources of ophthalmic nosocomial outbreaks commonly linked to adenovirus types 8 and 19. Desiccated virus remains viable and can be recovered after 49 days on dried plastic or metal surfaces.1
Areas that can Benefit from Sterile Diaton tonometer:
- Emergency departments
- Urgent care centers
- Ophthalmology clinics, optometrist offices, and procedure rooms
- Neonatal intensive care units (NICUs)
“Items that touch mucous membranes — such as the eye — must be, at minimum, high-level disinfected. Items that contact or enter sterile tissues — such as instruments that are used for surgical procedures — or touch an ulcerated cornea must be sterilized. ”
The Joint Commission, Division of Healthcare Improvement
FDA cleared Scleral and Transpalpebral tonometry technology shows indisputable advantages vs All other contact and corneal tonometers which require direct contact with the cornea. Since there is No Contact with an eyeball, Risk of Infection or Cross Contamination is Null with Diaton’s technology.
Another Key Item is being overlooked in ED ER Setting is that variability in Corneal Biomechanics influences All other corneal or contact devices, but not the Diaton:
Diaton measures Accurate IOP in Eyes that are affected by corneal pathologies which make corneal tonometry inaccurate, and often impossible – corneal injury, corneal edema, keratoconus, atypical corneal thickness, corneal rigidity, keratitis, cornea dimness, post keratoplastics, keratoprosthesis, post LASIK, high degree of ametropy, astigmatism and many other conditions which walk into Emergency Departments and Emergency Rooms.
Diaton tonometer can measure accurate IOP with contact or scleral lenses still being on the eye.
Diaton tonometer is priced very competitively and is delivered complete with 2 year warranty, a carry case, calibration & training plate, battery, and training DVD in 5 different languages, manuals, quick starter guide, plus unlimited phone based training and support. To contact the company dial 1-877-342-8667 or Order Tonometer Diaton online at
https://tonometerdiaton.com/order-information-diaton-tonometer-price/
Who Uses the Diaton Tonometer?
Diaton Tonometer is intended for use by Ophthalmologists, Optometrists, Ophthalmic technicians as well as Inpatient & Outpatient Clinics such as Hospitals, Emergency Rooms, Urgent Care Clinics, Nursing & Elderly Homes, General & Specialty Practitioners.
About DevelopAll Inc.,
DevelopAll Inc – home of Diaton Tonometer, is committed to the global fight against blindness caused by glaucoma. A unique team of engineers, medical, legal and business experts makes DevelopAll Inc. uniquely placed to provide DIATON diagnostic tonometer, which measures intraocular pressure (IOP) through the eyelid and sclera, making it possible to diagnose glaucoma on the early stage and appoint necessary treatment and medicines and provide a much more favorable outcome for the patient. More about DIATON Tonometer Glaucoma Eye Test at http://www.Diaton.com
Contact: Alina Lagoviyer Media / Public Relations 1-877-342-8667 ext 707
DevelopAll Inc., 272 Hull Avenue, Staten Island, NY 10306
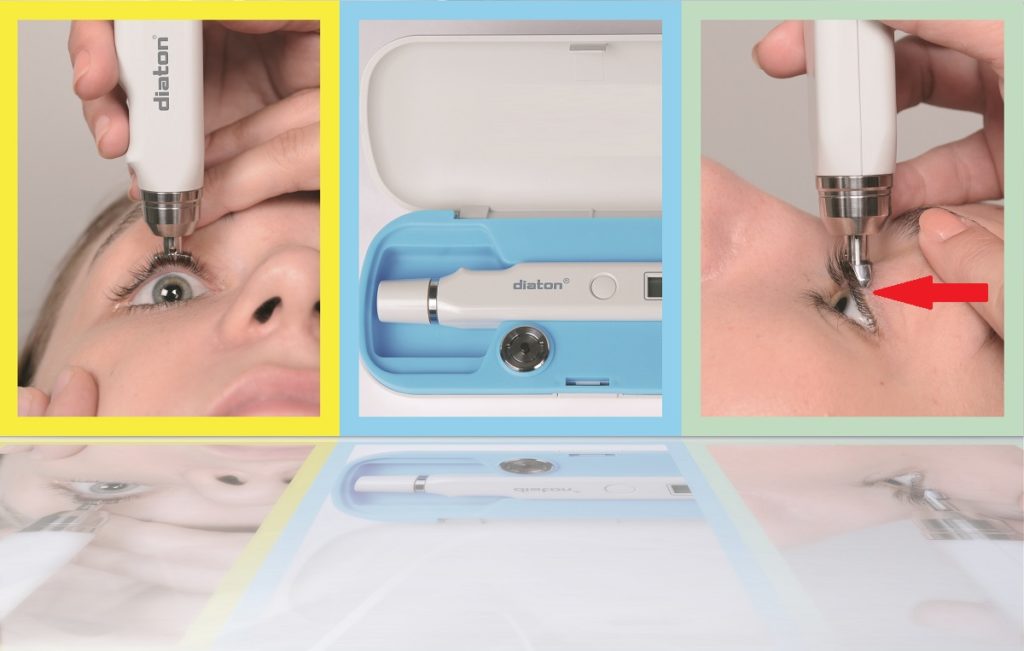
One Tonometer – Many Applications!