Embracing Innovation in Glaucoma Screening: The Diaton Digital Tonometer Revolution
The landscape of glaucoma screening is witnessing a transformative shift with the introduction of the Diaton Digital Tonometer by DevelopAll Inc., based in New York (originally BiCOM). This cutting-edge device is redefining intraocular pressure (IOP) measurement with its non-invasive approach, offering a significant leap forward from traditional methods that often grapple with the variability of the cornea affecting accuracy.

The Corneal Challenge in IOP Measurement
Current IOP measurement techniques are subject to numerous variables that can compromise accuracy, including variations in corneal thickness, rigidity, and conditions such as keratoconus, edema, and scarring from surgeries or injuries. The Diaton Tonometer emerges as a pivotal solution, operating independently of the cornea to provide more accurate and flexible IOP measurements.
The Diaton Difference
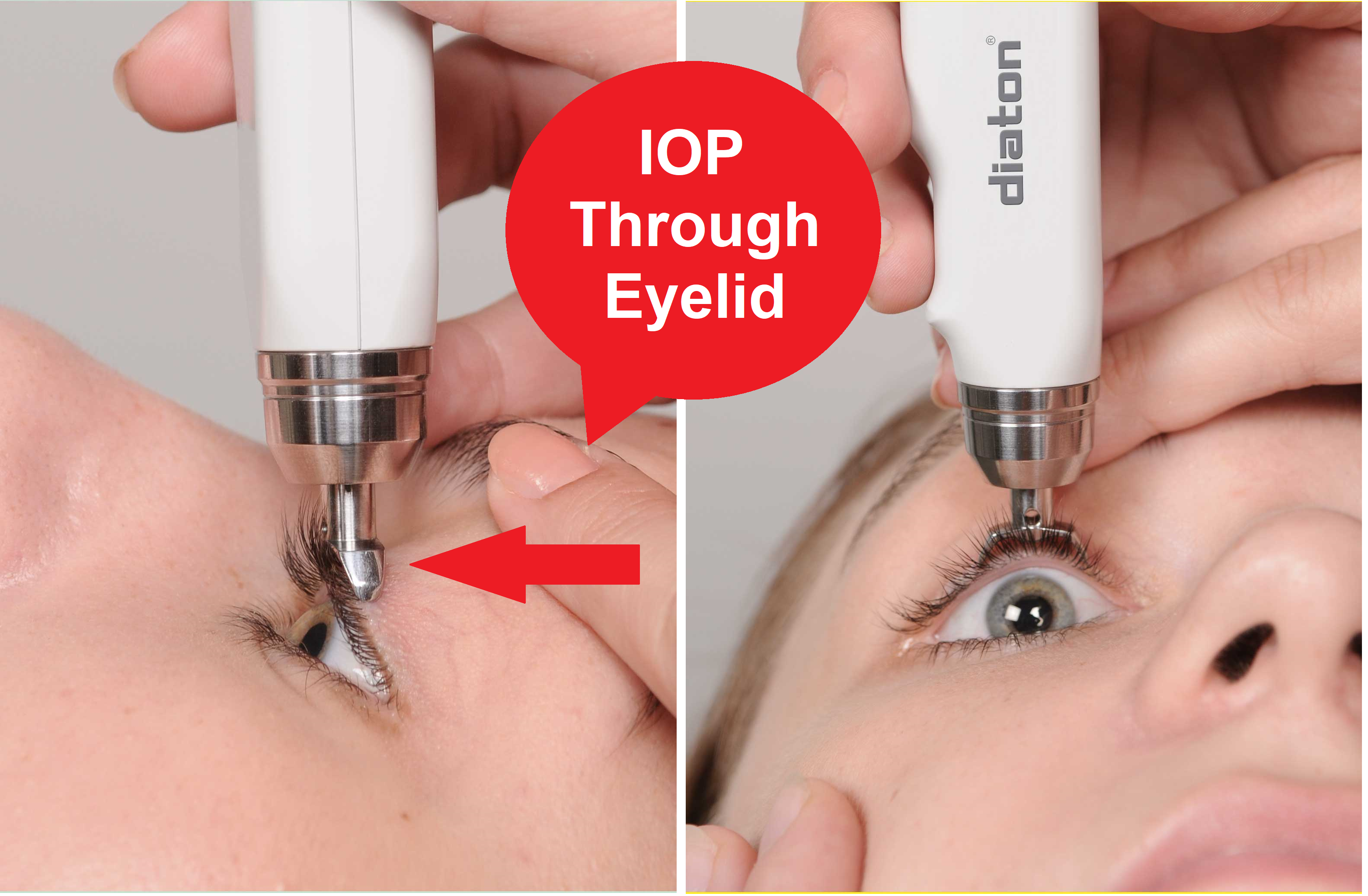
Crafted by DevelopAll Inc., the Diaton is a digital, non-contact tonometer that measures IOP through the upper eyelid and above the limbus. This innovative approach caters to patients with atypical corneas, offering a more accurate measurement free from the influence of corneal variability. Roman Iospa, CEO of DevelopAll Inc., emphasizes the device’s precision and its ability to navigate the complexities of corneal characteristics.
Advancements and Applications
The FDA-approved Diaton Tonometer is a transpalpebral and transscleral instrument that does not require anesthesia or sterilization. Its pen-like design enhances portability and ease of use, allowing for measurements without removing contact or scleral lenses. The device stands out for its lack of consumables, absence of calibration needs, and the elimination of pachymetry adjustments for central corneal thickness. Its non-invasive nature minimizes the risk of corneal abrasion and infection transmission, a crucial advantage during health crises like the COVID-19 pandemic.
A Safer Choice During Health Crises
In the wake of the coronavirus pandemic, the Diaton Tonometer has proven its worth as a safer option, minimizing the risk of viral transmission through non-contact measurements. Emil William Chynn, MD, MBA, FACS, from Park Avenue Laser Vision in New York, highlights the tonometer’s utility in challenging cases, including pediatric patients, those with refractive surgeries, and individuals with corneal pathologies.
Technique and Training
The Diaton Tonometer employs a straightforward technique, using a surgical-grade stainless steel tip that measures IOP via the eyelid. This process ensures accurate readings without the need for direct corneal contact, supported by a digital display for immediate results. DevelopAll Inc. provides comprehensive online support, facilitating integration into clinical practice and reducing the learning curve for staff.
Enhancing Clinical Efficiency
Mark Latina, MD, an associate clinical professor at Tufts University School of Medicine, praises the tonometer for its ease of use, reproducibility, and the ability to streamline the examination process. The device’s efficiency, combined with the absence of consumable parts and the need for a slit lamp, significantly reduces examination times.
Continued Interest and Adoption
Despite being on the market for a decade, the Diaton Tonometer continues to garner attention for its innovative approach to IOP measurement. Its ability to provide consistent and accurate readings is reshaping glaucoma screening and management, earning accolades from eye care professionals worldwide.
As the eye care community embraces technological advancements, the Diaton Digital Tonometer stands as a testament to innovation, safety, and precision in glaucoma screening, promising a future where eye care is more accessible, accurate, and patient-friendly.
Visit Diaton tonometer website for additional details or to order: www.TonometerDiaton.com