Unique Diaton Tonometer Featured at the American Academy of Ophthalmology (AAO) 2024 in Chicago
Revolutionary Non-Invasive Device Delivers Intraocular Pressure (IOP) Measurement Independent of Corneal Variables
Chicago, IL – The groundbreaking Diaton Tonometer, a unique non-invasive device for intraocular pressure (IOP) measurement, is being showcased at the prestigious American Academy of Ophthalmology (AAO) 2024 event in Chicago. Recognized for its advanced transpalpebral and trans-scleral technology, the Diaton Tonometer offers ophthalmologists a safer, more efficient method to measure IOP without the need for corneal contact—providing distinct advantages over traditional tonometry methods.

The Diaton Advantage: Independent of Corneal Biometrics
Unlike traditional tonometry devices, which rely on corneal contact and are influenced by corneal thickness, curvature, or biomechanics, the Diaton Tonometer measures IOP through the upper eyelid and sclera. This key distinction allows the Diaton to provide accurate readings without the need to adjust for corneal properties, including central corneal thickness (CCT). In clinical settings, CCT variations can significantly affect the accuracy of IOP measurements, leading to under- or over-estimations that can complicate glaucoma diagnosis and management.
By bypassing the cornea entirely, the Diaton Tonometer eliminates these concerns, providing a more reliable IOP reading across all patients. This is especially beneficial in situations where corneal conditions, surgeries, or abnormalities, such as keratoconus or post-LASIK surgery, make traditional tonometry methods unreliable.
Addressing Critical Eye Conditions: The Only Solution in Certain Cases
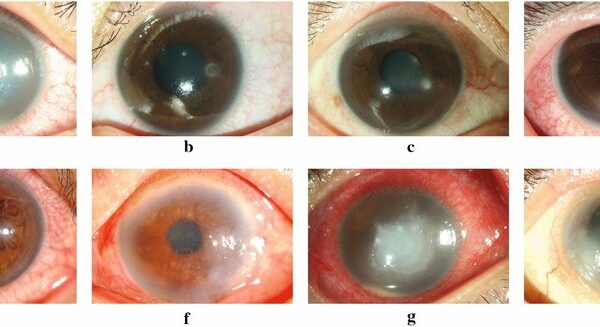
The Diaton Tonometer is the only solution in several eye conditions where corneal-based tonometry methods are either ineffective or inappropriate. For example, in patients with:
- Keratoconus: A progressive thinning and bulging of the cornea, keratoconus distorts traditional IOP readings due to the irregular corneal shape and thinness. The Diaton tonometer, unaffected by corneal properties, provides an accurate IOP reading without the need for complex adjustments.
- Post-LASIK and Corneal Surgery: Patients who have undergone LASIK or other refractive surgeries often present with thinner or altered corneas, making traditional IOP measurement less accurate. The Diaton tonometer allows clinicians to monitor IOP without being affected by these corneal changes, ensuring accurate and consistent pressure readings.
- Ocular Surface Disease: In patients with severe dry eye, corneal scars, or recurrent infections, corneal tonometry can cause discomfort and risk further irritation. The Diaton’s transpalpebral approach ensures that these patients can have their IOP measured safely, without exacerbating their condition.
- Contact Lens and Scleral Lens Wearers: Traditional tonometry requires the removal of lenses before measuring IOP, but the Diaton tonometer can be used while scleral lenses remain in place. Studies have shown that scleral lenses may elevate IOP while on the eye, posing a particular risk for glaucoma patients. The Diaton tonometer allows for IOP to be measured accurately without needing to remove the lenses, offering real-time monitoring and minimizing risks for these patients.
A Safer and More Efficient Solution for Glaucoma and Other Eye Conditions
The Diaton Tonometer is revolutionizing the way ophthalmologists, optometrists, and emergency departments approach IOP measurement. With its non-invasive nature, the device reduces the risk of infection, corneal abrasions, and patient discomfort—all of which are associated with traditional tonometry methods. Moreover, the device requires no numbing drops, no replacement tips, and no constant recalibration between patients, streamlining workflow and saving time.
“At the AAO 2024, we’re excited to showcase how the Diaton Tonometer can benefit healthcare professionals and their patients by providing a safer, faster, and more reliable solution for IOP measurement,” said Roman Iospa, CEO of DevelopAll, Inc., the company behind the Diaton Tonometer. “For patients with complex eye conditions, the Diaton is often the only solution that delivers accurate results without the limitations of corneal-based devices.”
Visit Diaton at AAO 2024 in Chicago
Attendees of AAO 2024 are invited to visit the Diaton booth for live demonstrations and to learn more about how this innovative tonometer can enhance patient care in clinical practice. Experts will be on hand to discuss the benefits of the device and answer any questions from healthcare professionals.
For more information about the Diaton Tonometer, visit www.TonometerDiaton.com.
About DevelopAll, Inc.
DevelopAll, Inc. is a leader in non-invasive medical technologies, specializing in innovative solutions for ophthalmology. The company’s flagship product, the Diaton Tonometer, has been adopted in healthcare institutions worldwide for its unique approach to measuring intraocular pressure and its effectiveness in the diagnosis and management of glaucoma.